Credit: Global Carbon Capture and Storage Institute
Previously, we talked about how Earth transfers naturally produced carbon between sky, sea, and soil.
Today, scientists are working to put CO2 from fossil-fuel combustion and agriculture back into the ground rather than into the atmosphere—by mimicking Earth’s natural carbon cycle.
Capturing and compressing CO2 from the exhaust stream of, say, a coal power plant, is challenging and expensive. But when done, the gas becomes a low-density liquid.
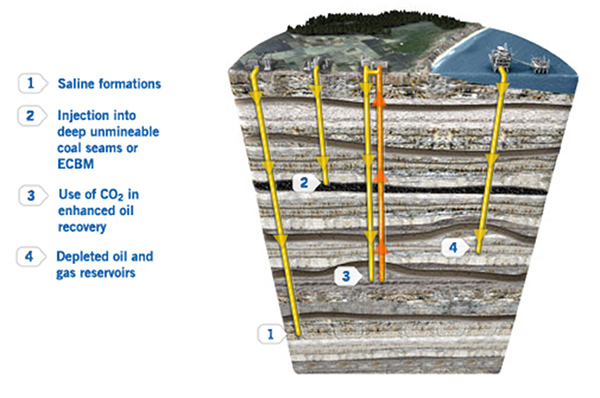
Researchers have developed and tested methods to pump it deep underground—into depleted oil fields, coal seams, and rock formations whose small pores are filled with saltwater.
Once there, it dissolves and the saltwater becomes carbonated, less than a soft drink. Studies suggest that carbon can remain trapped in these formations indefinitely, similar to the way hydrocarbons are trapped.
While international tests of these processes have been successful, other more experimental methods are striving to turn CO2 gas into useful solid materials.
New trials have injected CO2 into volcanic basalt, where it formed carbonate minerals.
Others combine carbon and calcium, similar to the way snails and clams draw them from seawater to make their shells.
The challenges with all these methods are expense and scale. Storing enough human-produced carbon to make a difference on the climate, in the time frame needed, will not be easy.
But thanks to government and industry investment in research, we now have enough experience to begin large-scale tests.
Background
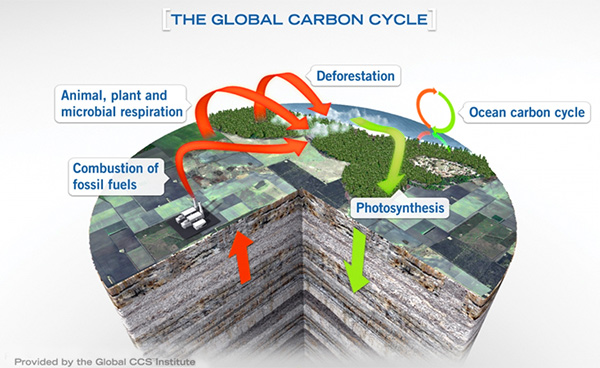
Synopsis: Carbon and oxygen just love to form compounds together, especially CO2. Earth sequesters carbon naturally by chemically stabilizing it or burying it so it can’t react with oxygen in the atmosphere. As we discussed in a previous EarthDate, these natural processes of carbon sequestration on Earth can give us some innovative ideas about how to reduce carbon dioxide in the atmosphere ourselves.
- Rapidly changing CO2 concentrations have been shown to alter the delicate balance of our atmosphere and impact global climate, oceans, and biotic life.
- When we burn fossil fuels, we enable carbon stored in the Earth’s crust for millions of years to chemically bond with oxygen.
- Over half of CO2 released into the atmosphere dissolves into the ocean over a period of decades to hundreds of years. The remainder contributes for much longer to atmospheric warming and other natural processes in the carbon cycle.
- When we deforest areas for agricultural purposes, less carbon is absorbed from the atmosphere and stored in the biosphere through photosynthesis.
- The process of keeping carbon from entering the atmosphere is called Carbon Capture and Sequestration, or Carbon Capture and Storage (CCS).
- Capturing, separating, and concentrating carbon dioxide is expensive but rapidly evolving technology. (We will talk about some of the specific ways this is done in a future EarthDate.)
- The most effective way to sequester carbon is to isolate it from the atmosphere by returning it to the subsurface, where it came from. This process, called geological sequestration, isolates CO2 in natural pore spaces in rocks, similar to how natural processes retain hydrocarbons in the subsurface.
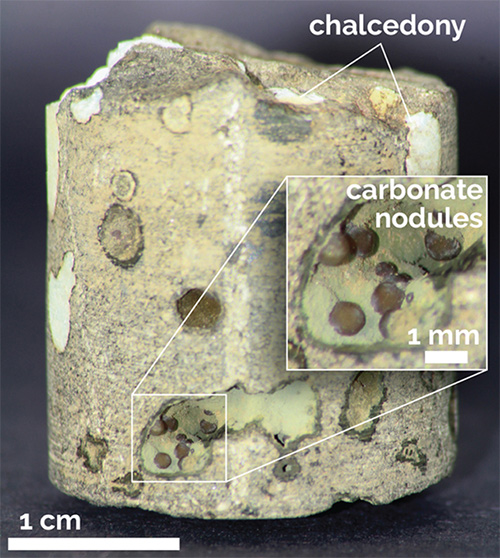
A core section from the Wallula Basalt Pilot Project in Washington State obtained 24 months after carbon dioxide injection shows carbonate nodules. Similar nodules from other depths in the core were identified as the carbonate mineral ankerite. Credit: American Chemical Society/Environmental Science and Technology Research Letters/Pacific Northwest National Laboratory - Researchers have studied CO2 storage in old depleted oil fields, saline formations, coal seams, and shale beds that lie more than a mile deep.
- CO2 becomes denser under the great pressures that naturally exist in Earth’s subsurface until it becomes a very low viscosity fluid less dense than water, which makes it disperse through pore spaces similar to how ordinary gases or liquids do.
- The safest place to store CO2 underground is below impermeable caprocks, similar to how oil and gas are trapped naturally in the subsurface prior to production and combustion.
- CO2 injection has been used for decades to help sweep the final droplets of oil out of old oilfields. Some of the CO2 remains in the ground after this process is complete, reducing the carbon intensity of oil produced this way.
- CO2 can be dissolved in produced saline water and re-injected into depleted oil fields.
- Extensive studies and calculations indicate that subsurface storage volumes can receive regional industrial emissions for many decades or longer.
- One important concern, however, is potential leakage of CO2 through natural faults and fractures and especially through cement in old oil-field wells. Extensive national and international testing to address this concern has occurred both onshore and offshore.
- Another way to isolate carbon from the atmosphere is to get it to form a stable compound.
- The biggest issue with this approach is that past studies have shown that it takes a lot of time (probably hundreds of years or longer) for the needed reactions to occur, and volumes sequestered this way are relatively small.
- Today there are two main approaches to this process.
- Carbon mineralization utilizes highly reactive volcanic basalts that react with carbon dioxide to trap it in carbonate minerals like calcite, dolomite, and ankerite. Scientists have experimented with injecting CO2 directly into basalts and have seen some iron carbonate, ankerite, precipitate after about 2 years.
- Mineral carbonation is another emerging commercially driven process that involves reacting magnesium or calcium oxides with CO2 to produce solid inorganic carbonates that can be used in cements.
- We take useful carbon out of subsurface rocks for our energy needs, so it makes sense that it’s possible to put back some of that carbon to minimize atmospheric emissions.

