
Credit: Alan Gerrard / Rusty lock, CC BY-SA 2.0, via Wikimedia Commons
If left unprotected, something made of iron will dissolve into dust. That’s because of rust.
Some metals, like gold and platinum, are naturally stable and keep their shiny appearance.
Other metals like aluminum, copper and silver develop patina or tarnish that protects the raw metal beneath.
Iron is different. It’s the most abundant element on Earth, followed by oxygen—together they make up over 60% of all elements. And they like to get together:
Almost all iron on the surface of Earth appears as an iron oxide—a mixture of iron and oxygen.
We smelt iron oxides to get pure iron, which we use as a metal or blend into alloys like steel. But once it’s been separated from oxygen, it wants to bond again.
When water gets involved, rust forms.
It’s not well defined as a chemical process, but initially the iron oxidizes, then water turns that molecule into a new compound we call rust, which replaces the metal.
And because the rust molecule is larger, it doesn’t fit in the same place and flakes off as rust. This exposes new metal, which will rust and flake off—until all the iron is gone.
With so much of our buildings, bridges, pipelines and other infrastructure made of iron or steel, it’s important to keep them oiled, painted, coated or alloyed to be protected.
Because as Neil Young said, rust never sleeps.
Background
Synopsis: Rust happens when water and oxygen get together with iron to form a reddish-brown compound that, if left untreated, will completely replace the metal and disappear as it gradually flakes away. With so much infrastructure dependent on iron and its alloys like steel, corrosion prevention is very important to civilization.
- Rust is the common name for the group of hydrated iron oxide minerals that form when iron is exposed to water and oxygen.
- With its two unpaired electrons, oxygen (O) is extremely reactive, so it combines with other chemicals to form more-stable compounds.
- Iron (Fe) forms compounds so readily with oxygen that it is rarely found in its pure form in nature. Humans refine iron ore to produce metallic iron.
- When water (H2O) gets into the mix, iron corrodes (oxidizes) to form rust, which flakes away because the new compound is larger than the iron it replaces.
- Iron and oxygen are the two most abundant elements on Earth, at 31.9% and 29.7%, respectively.
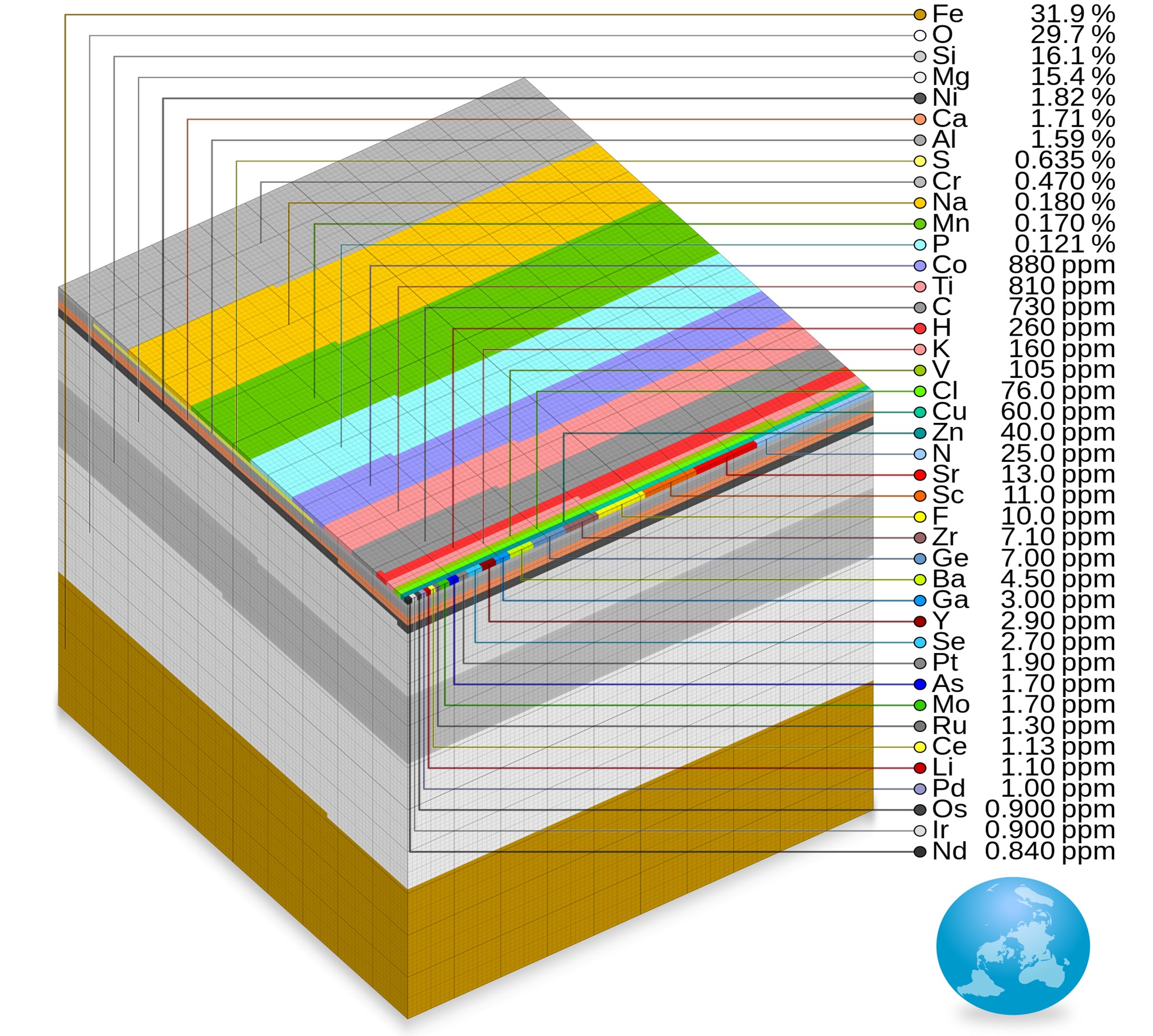
Parts-per-million cube showing relative abundance of elements by mass for the entire Earth. The mass of the Earth is approximately 5.97×1,024 kg. Cumulatively, iron (31.9%), oxygen (29.7%), silicon (16.2%) and magnesium (15.4%) make up 93.2% of Earth’s mass.
Credit: Cmglee, CC BY-SA 4.0, via Wikimedia Commons- Iron makes up about 85.5% of Earth’s core.
- Oxygen is the most abundant element in Earth’s crust (46.1%), followed by silicon (28.2%), then aluminum (8.2%) then iron (5.6%).
- The sheer abundance of both elements creates plenty of opportunities for iron and oxygen to react on Earth’s surface.
- The reason that iron is so plentiful goes back to the formation of the first stars and iron’s position at the tipping point between energy-producing fusion and fission reactions. (ED-091 Our Most Common Element). Iron is the heaviest element produced during fusion without having to add energy, and it is the lightest element produced during fission without having to add energy. Energy-wise, everything in the universe wants to end up as iron!
- On most planets, available oxygen was used up long ago and sequestered into rocks, but Earth has a source of oxygen generated by photosynthetic plants, plankton and bacteria. Photosynthesis uses sunlight, carbon dioxide and water to produce energy, with oxygen as a “waste” product.
- Photosynthetic bacteria started producing oxygen on Earth more than 2 billion years ago, so excess oxygen has built up to compose about 21% of our atmosphere (ED-228 Flammable Planet). Without photosynthetic organisms, Earth would be a much different place!
- As a transition metal, iron has a number of number of different ways to corrode, chemically speaking, which are called oxidation states. The most common of these are ferric ions with a +3 charge and ferrous ions with a +2 charge.
- Ferric compounds tend to occur when oxygen is abundant in oxidizing environments, while ferrous compounds form when oxygen is scarce (reducing environments).
- Pure iron oxides like ferric hematite (Fe2O3) and ferrous magnetite (Fe3O4) are not rust. Rust will not form in dry air.
- Rust is not well defined in chemistry, but what we usually mean when we say rust is some type of hydrated (containing H2O) ferric iron oxide with a red or reddish-brown color. When rust forms, two electrochemical reactions take place simultaneously.
- At an anode (a point with a positive charge), iron releases electrons to form positively charged iron ions: 2Fe → 2Fe2+ + 4e-
- At a cathode (a point with a negative charge), water and oxygen combine to form negatively charged hydroxide:O2 + 2H2O + 4e- → 4OH-
- Then the iron and hydroxide ions react in the water to form iron hydroxide: 2Fe2+ + 4OH- → 2Fe(OH)2
- And finally, the iron oxide reacts with oxygen to yield rust, Fe2O3.H2O.
- If the water is salty or acidic it is an even better electrolyte, and the reaction proceeds even more vigorously.
- While oxides protect some metals by making their surfaces more chemically stable, rust does not protect metallic iron or its alloys.
- Some metals, like gold and platinum, are chemically stable, forming pure metallic veins in nature.
- Other metals, like copper, silver and aluminum, once refined, form oxide coatings that are chemically stable, sealing and protecting them from the elements.
- We refer to the dark oxidation of silver as tarnish and hand-polish our silver keepsakes to keep them shiny and bright.
- The oxidation of copper is called patina or verdigris; this is the coating that creates the pale green color of the copper Statue of Liberty.
- Aluminum oxide creates a dull stable coating on metallic aluminum.
- Unlike other metals, hydrated ferric iron oxides do not bond to the underlying metallic iron.

Layers of rust flake off of exposed iron metal because the hydrous ferric oxide molecules are larger than the iron atoms they replace, so they can’t fit back into the space left by dissolution of the iron atoms.
Credit: Laitr Keiows, CC BY-SA 3.0, via Wikimedia Commons - The larger hydrated molecules can’t fit back into the space previously occupied by iron atoms, so they pull away from the surface and flake off, exposing more metallic iron to the elements.
- Ultimately this process can completely consume the underlying metal.

The fender of this car has rusted through, probably because of salting to melt ice on roads.
Credit: Cameron Hill, CC0, via Wikimedia Commons
- One way to prevent rust is to protect iron surfaces from air and moisture.
- Iron can be oiled, painted or powder coated to prevent rust, as long as the metal is completely sealed.
- Iron may be protected by coating with zinc in a process called galvanizing or coating with magnetite in a process called bluing.
- Iron may be alloyed with other metals that cover its surface with a very thin layer of oxide. Stainless steel is an example that contains chromium that oxidizes to chromium oxide, sealing the steel and preventing rust.
- But if iron or its alloys must be exposed to the elements, like in pipelines, bridges, ships or water heaters, a galvanic cathodic protective system can be employed.
- Cathodic protection sacrifices a more electropositive element, like zinc, magnesium or aluminum, to corrosion.
- These metals are attached as galvanic anodes that do not require an external source of power. They naturally give up their electrons more readily than iron, slowing or stopping the process of iron rusting.

This worn galvanic anode (also known as a sacrificial anode) protects the steel of a yacht's propeller shaft. Material is lost from the anode rather than the metal of the shaft, protecting the shaft from rusting.
Credit: Springnuts, CC BY-SA 4.0, via Wikimedia Commons

