Credit: NOAA
Ozone makes up a tiny portion of Earth’s atmosphere, but it allows for life on Earth.
The oxygen we breathe is what’s called molecular oxygen, O2—two atoms of oxygen bonded together. High above Earth’s surface, in the stratosphere, the Sun’s intense radiation splits those oxygen molecules, and a tiny fraction reform as ozone, or O3—three oxygen atoms, with a very important property.
Ozone absorbs UV-C, a form of ultraviolet light so dangerous, it can destroy microbes and alter DNA in larger lifeforms. If Earth weren’t protected by its ozone layer, scientists think UV-C would sterilize its surface, perhaps wiping out surface life.
But ozone is vulnerable. In the 1930s, aiming to replace more dangerous refrigerants, scientists developed chlorofluorocarbons, or CFCs. They proliferated in cooling systems around the world.
But the chlorine in CFCs, when it entered the atmosphere, bonded with free oxygen and disrupted ozone formation.
Deeply worried about UV-C damage, scientists convinced politicians of the threat, and in 1987, the Montreal Protocol was signed by every nation on Earth.
Since then, countries have phased out CFCs, and the ozone layer is rebounding. It should return to pre-CFC levels in 30 years.
A positive example of how humans can unite to solve environmental problems.
Background
Synopsis: In the mid-1980s, humanity got a wake-up call that mobilized a generation of scientists to fight a threat to Earth’s protective sun shield, our fragile stratospheric ozone layer. Depletion of the ozone layer above Antarctica raised alarms, but in 1987, scientists and governments worked together on a solution to ban ozone-depleting substances, the Montreal Protocol. In 2023, scientists assessed a bright future for healing Earth’s ozone hole.
- Allotropes are different structural forms of the same element that occur in the same physical state. Atomic oxygen (O), molecular or diatomic oxygen (O2) and ozone or triatomic oxygen (O3) are three gaseous allotropes of oxygen.
- In 1776, Antoine Lavoisier proved oxygen was an element with an atomic number of 8.
- Eighteenth-century scientists noted that passing electricity through oxygen produced a pungent odor, and in the late 1830s, the gas was named “ozone” after the Greek word ozein, meaning “to smell.”
- By 1865, scientists had figured out that ozone is a gas that occurs in trace amounts in Earth’s atmosphere, consisting of three oxygen atoms, O3.
- In the early twentieth century, scientists discovered that the distribution of ozone in the atmosphere varies and that ozone plays an important role for life on Earth.
- In 1899, French scientists Fabry and Perot developed an interferometer for astrophysical applications.
- In 1913, Fabry and Buisson adapted the interferometer to measure ozone in the atmosphere.
- In the mid-1930s, scientists used ozonesonde balloons to demonstrate that ozone concentrations increase in the stratosphere 12 to 18 mi (20–30 km) above Earth’s surface.
- They also showed that although it is dispersed in very low concentrations, ozone absorbs ultraviolet radiation in the atmosphere, a global sunscreen.
- In 1924, Oxford meteorologist Gordon Dobson invented the Dobson Ozone Spectrophotometer (Dobsonmeter) to measure total atmospheric ozone from the ground. A global network of these instruments was established in 1957.
- Dobson developed a standard measurement of trace gases in the atmosphere called Dobson Units (DU). A DU is the micrometer thickness that a proportional layer of a particular pure gas would occupy in an atmospheric column at sea level.
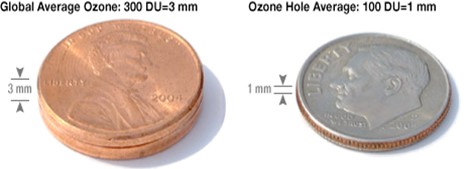
Compressed to sea level pressure, the ozone in the atmosphere would form a layer about the height of two pennies stacked together. Levels in the ozone hole are much less—only the height of a single dime.
Credit: NASA Ozone Watch - If all the air in a column from the ground surface to the edge of space (62 mi thick [100 km]) was compressed to sea level pressure (1 atmosphere [101 kPa]) at 32°F (0°C), the column would be 5 mi (8 km) tall.
- But the ozone in that column would compress down to just one-eighth inch (3 mm, 300 DU), about the height of two American pennies. So delicate!
- Dobson developed a standard measurement of trace gases in the atmosphere called Dobson Units (DU). A DU is the micrometer thickness that a proportional layer of a particular pure gas would occupy in an atmospheric column at sea level.
- This tiny amount of ozone dispersed in the atmosphere is vitally important to life on Earth because it forms an invisible protective shield that absorbs harmful ultraviolet radiation from the Sun.
- Ultraviolet light comes in three types determined by the wavelength of the radiation: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm).
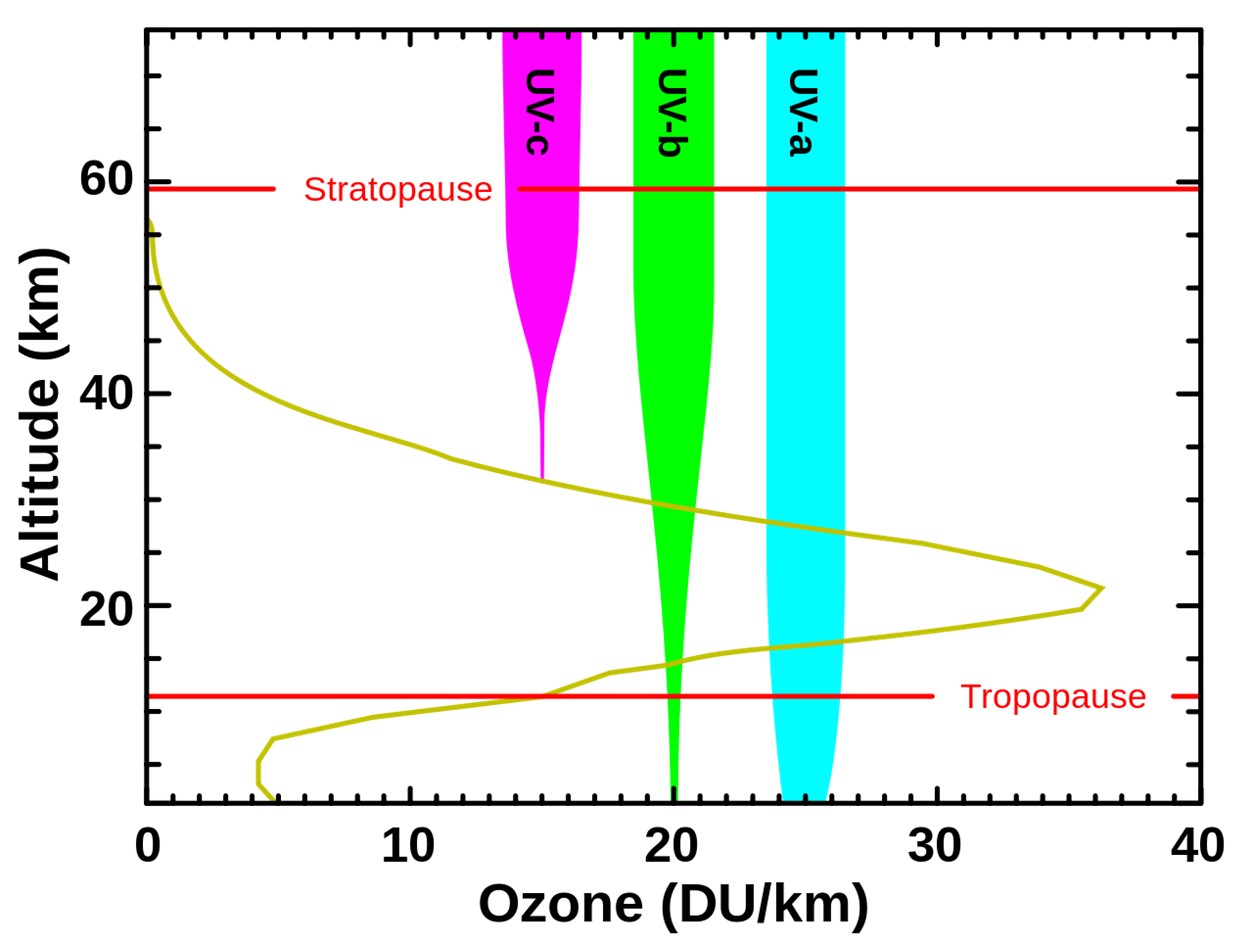
Levels of ozone at various altitudes (DU/km) and related stratospheric blocking of several types of ultraviolet radiation.
Credit: NASA, public domain, via Wikimedia Commons - With the shortest wavelength, UV-C is the most damaging to DNA, so it is also known as germicidal UV, because it can kill microbes. Thankfully, it is completely filtered by Earth’s ozone layer at an altitude of more than 18 mi (30 km).
- Medium-wavelength UV-B is biologically dangerous and can also produce DNA mutations, but it can’t penetrate beyond a few skin layers. Some UV-B penetrates the ozone layer and produces sunburn, cataracts, and skin cancer in humans.
- Most of the longer-wavelength UV-A that the Sun sends our way reaches Earth’s surface but is not as genetically damaging, so is less worrying.
- Ultraviolet light comes in three types determined by the wavelength of the radiation: UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm).
- In the 1930s, scientists figured out how ozone forms in the atmosphere and why 90% of it occurs in the stratosphere.
- Solar ultraviolet radiation constantly interacts with molecular oxygen (O2) in Earth’s atmosphere to create oxygen ions that combine with O2 to produce ozone (O3), but if other molecules are close by, the ozone tends to be destroyed as it participates in other chemical reactions.
- The delicate balance between this creation and destruction of ozone is governed primarily by the density of molecules in the atmosphere, and the sweet spot for ozone occurs within the stratosphere at about 15.5 mi (25 km) altitude.
- Also in the 1930s, the Frigidaire Division of General Motors began to develop applications for a new chemical synthesized by American chemical engineer Thomas Midgley, a chlorofluorocarbon (CFC) better known as Freon-12.
- Chlorofluorocarbons were developed to replace earlier refrigerants that were toxic, flammable or explosive, including ammonia, chloromethane, propane, methyl formate and sulfur dioxide.
- Their use as refrigerants expanded dramatically in the late 1950s, and other applications were discovered, such as their use as a propellant for aerosol cans.

Aerosol spray cans used CFCs as a propellant until the 1987 Montreal Protocol banned use. Air conditioners and refrigerators also used the compound, which has since been replaced with less-harmful propellants.
Credit: Andrew Magill from Boulder, USA, CC BY 2.0, via Wikimedia Commons
- Around 1973, scientists recognized that CFCs released into the atmosphere via aerosols and refrigerants could potentially impact the ozone layer. The discovery earned Paul Crutzen, Mario Molina, and F. Sherwood Rowland a Nobel Prize in 1995.
- Normally in the stratosphere, there exists a balanced process of both destruction and formation of oxygen (O2) and ozone (O3). UV light splits the oxygen molecule into two atoms of oxygen. Each of these atoms then bond with other oxygen molecules to form 2 ozone molecules. 3 O2 → 2 O3 (in the presence of UV light)
- When CFC’s are present, UV light causes the bond with the chlorine atom to break, leaving a chlorine radical. The free chlorine radical reacts with an ozone molecule in the stratosphere forming ClO (chlorine monoxide) and O2 (oxygen molecule). This lowers the concentration of ozone available for the natural reaction with oxygen. One free chlorine radical can react with as many as 100,000 ozone molecules before another reaction removes it from the atmosphere.
- For more detail about this complex chemical process, see these notes.
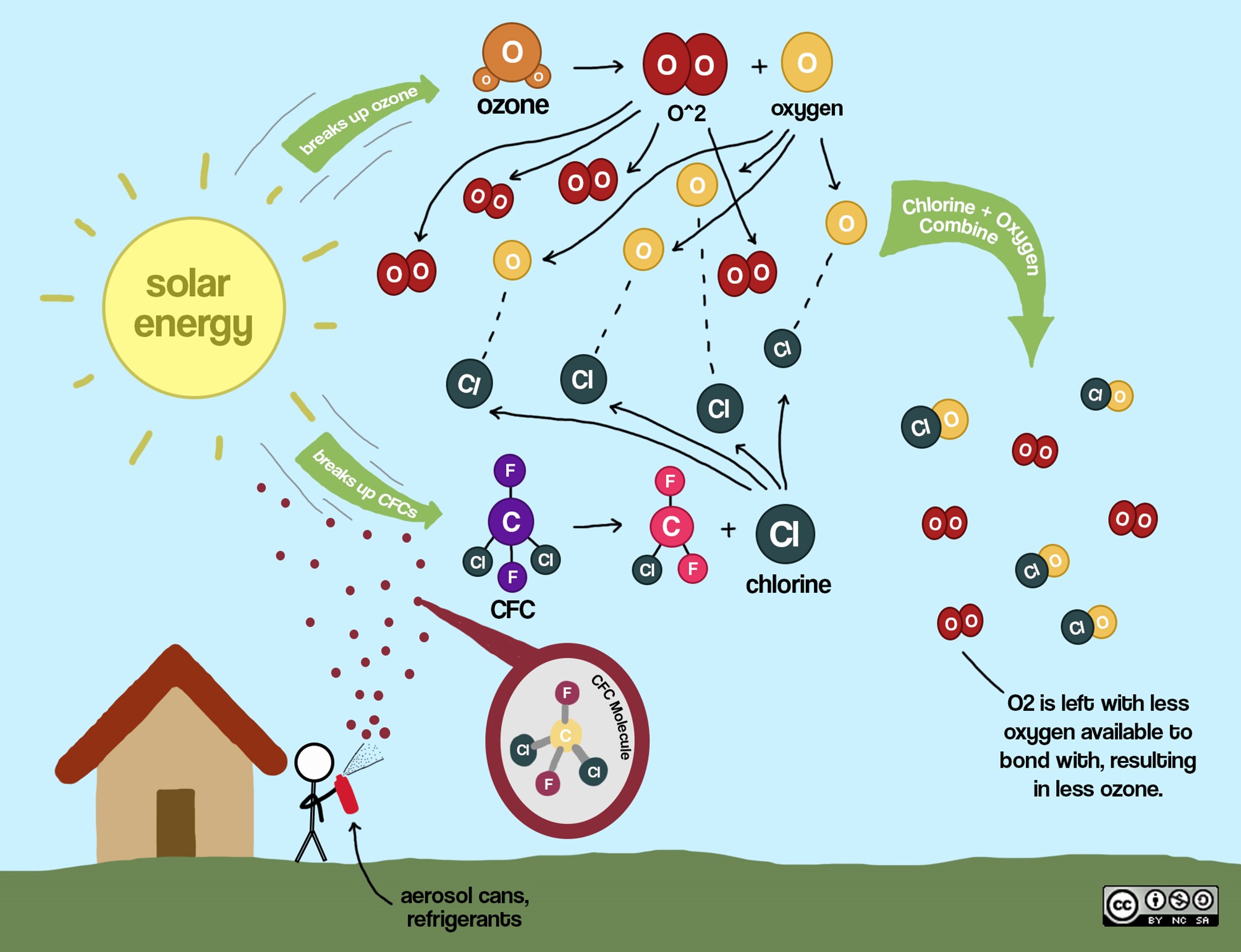
Chlorofluorocarbons released into the atmosphere are broken down by sunlight, releasing lone chlorine (Cl) ions in the atmosphere. These Cl ions bond with atomic oxygen (O) that forms when sunlight breaks down oxygen molecules. This leaves less atomic oxygen available to bond with diatomic oxygen (O2) to create ozone (O3) molecules. As a result, ozone concentrations become depleted.
Credit: Nicole Leihe, CC BY-SA 4.0, via Wikimedia Commons
- In 1985, scientists from the British Antarctic Survey using Dobson Ozone Spectrophotometers documented dramatically decreasing ozone levels each spring over Antarctica’s Halley Bay.
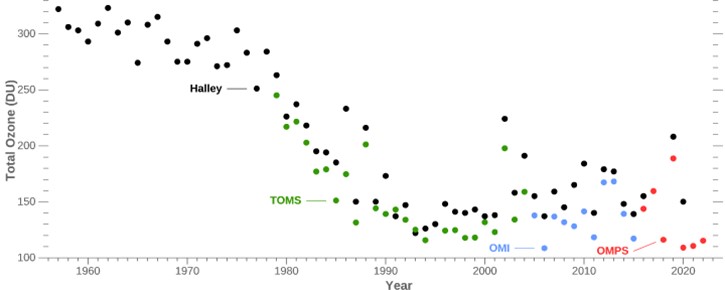
Beginning in the early 1980s, scientists measured a 66% drop in total atmospheric ozone over Antarctica each October. Ground-based instruments (black dots) and airborne instruments are used (green = Total Ozone Mapping Spectrometer [TOMS], Blue = Ozone Monitoring Instrument [OMI], and red = Ozone Mapping and Profiler Suite [OMPS]). The precipitous plunge has now stabilized as a result of global cooperation under the 1987 Montreal Protocol.
Credit: NASA- The depletion is localized over the South Pole because of polar stratospheric clouds (PSCs) that form during very low winter temperatures and are trapped by the strong circulation of the polar vortex.
- The frigid cloud particles attract chlorine and bromine that are available for reaction when the clouds warm in the Southern Hemisphere’s springtime, around October of each year. Higher chlorine and bromine concentrations react with atomic oxygen, leaving less available to form ozone.
- These effects can also occur in the Arctic but are less widespread because the North Pole stratospheric temperatures don’t get as cold.
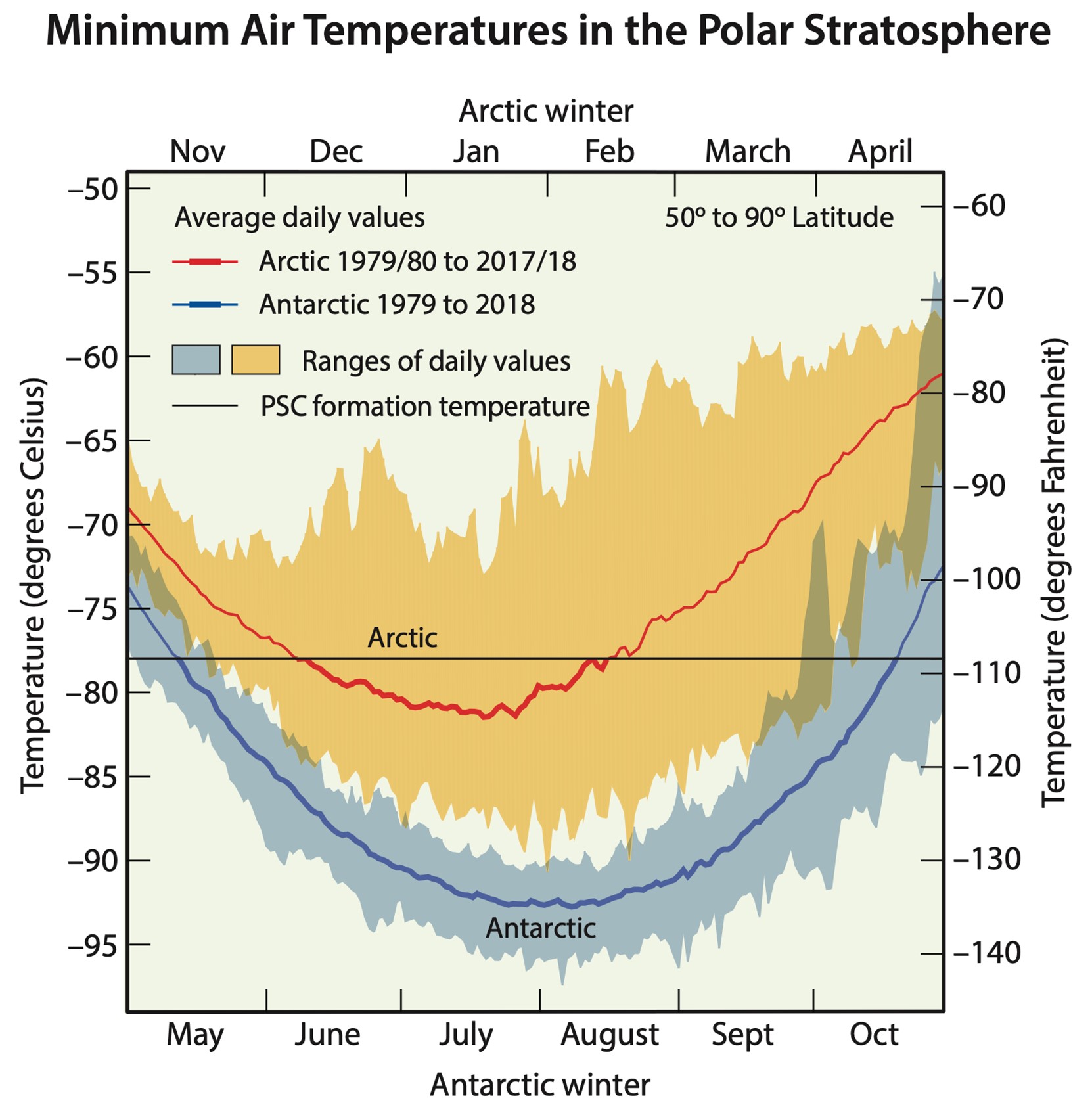
Extremely cold temperatures in the polar regions of Earth’s stratosphere cause the formation of polar stratospheric clouds, which attract chlorine and bromine. These chemicals become available for reactions when springtime warming occurs.
Credit : NOAA
- This depletion was dubbed the “ozone hole” although it isn’t really an empty hole, it is an area where ozone concentrations are depleted compared to the atmospheric average, allowing increased ultraviolet radiation to reach the ground.
- NASA’s OzoneWatch website states that without ozone in the atmosphere, “the Sun's intense UV rays would sterilize the Earth's surface.”
- The average concentration of ozone in Earth’s atmosphere is about 300 DU.
- Prior to the 1980s, the levels of ozone measured in the Antarctic atmosphere were all greater than 220 DU throughout the year.
- Scientists consider levels below 220 to be depleted, and the boundary of the ozone hole is defined by that concentration.
- Ozone levels average around 100 DU within the ozone hole.
- In the mid-1980s, concern about the ozone hole was widespread. Scientists convinced the public of potential imminent risks and rallied to address the challenge.
- Some worried that depletion of ozone levels over Antarctica would put Antarctic explorers at risk.
- Others feared that the expansion of the ozone hole would eventually allow harmful UV radiation to reach much of Earth’s surface, threatening the future of all lifeforms on Earth’s surface.
- Governments and scientists embraced the challenge and worked together to produce a solution—the 1987 Montreal Protocol.
- Signed on September 16, 1987, and enforced starting on January 1, 1989, this international treaty phased out the production and consumption of CFCs and certain other ozone-depleting substances (ODS) by 1996 in three steps.
- The first universally ratified treaty in UN history, every country on the planet ratified it and agreed to work together for the benefit of humanity.
- Former UN Secretary-General Kofi Annan called it “perhaps the single most successful international agreement to date.”
- It has undergone nine revisions, while later amendments address phase-out of additional varieties of ODS.
- Global public health savings are estimated at more than $1 trillion dollars since the inception of the Montreal Protocol.
- It is credited with the avoidance of around 280 million cases of skin cancer, 1.6 million skin cancer deaths and at least 45 million cases of cataracts.
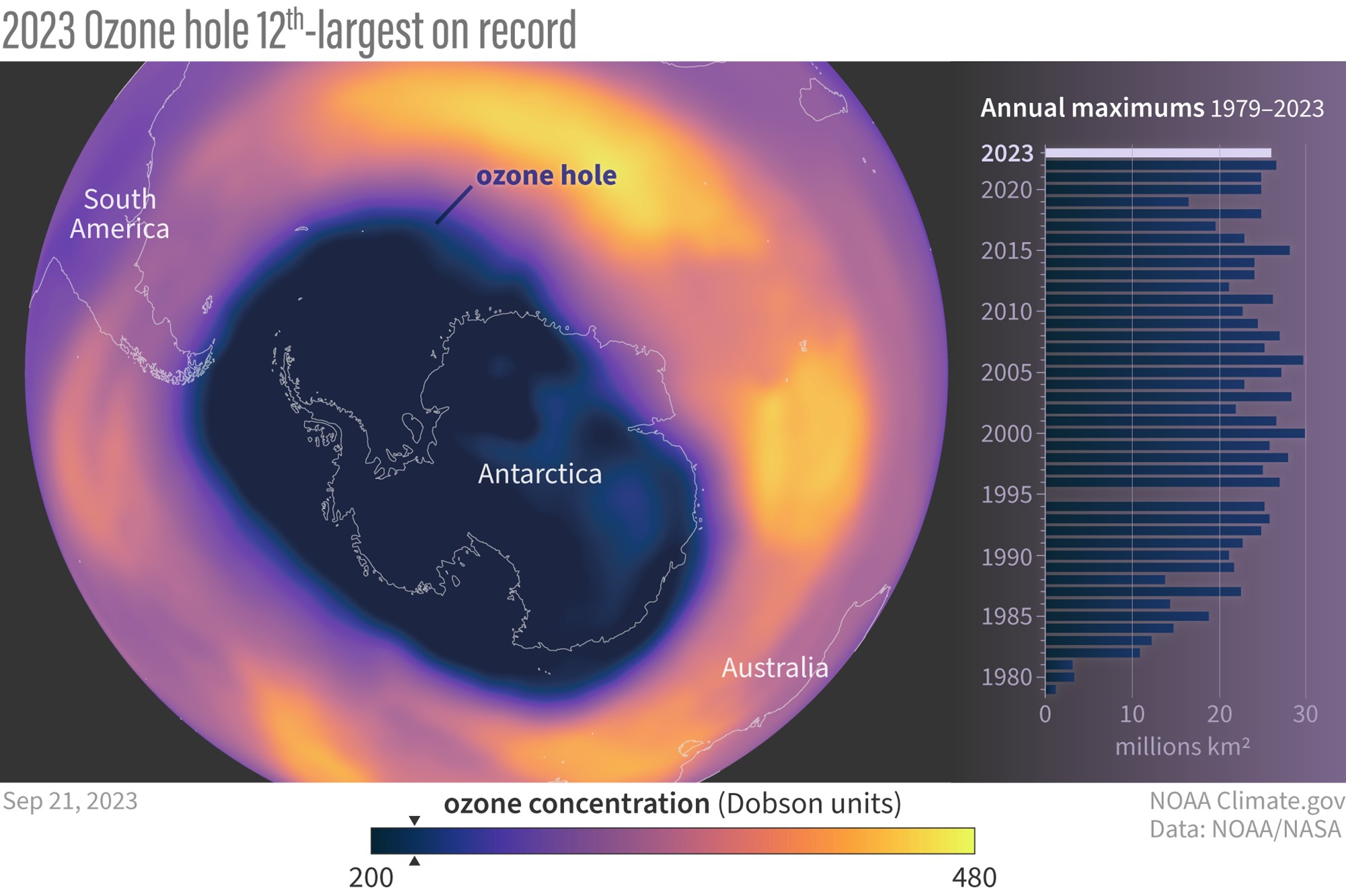
- Today, ozone levels have stabilized, and scientists expect that the “hole” in the ozone layer will heal to pre-1980 levels in the 2060s. However, natural disasters like volcanic eruptions may cause setbacks.
- CFCs have long atmospheric lifetimes, some lasting as long as 100 years, so recovery will take many decades.
- In 2019, the size of the ozone hole was the smallest seen since 1988, generating optimism.
- However, the 2023 ozone hole was the 12th-largest since measurement began in 1979, with ozone depletion starting earlier than usual. This is tied to the January 15, 2022, eruption of Hunga Tonga–Hunga Haʻapai (ED 298 Tonga: A Year Later), which shot huge plumes of saltwater vapor (containing chlorine atoms in sea salt, NaCl) into the stratosphere. Active stratospheric mixing during the year ameliorated the impact somewhat.
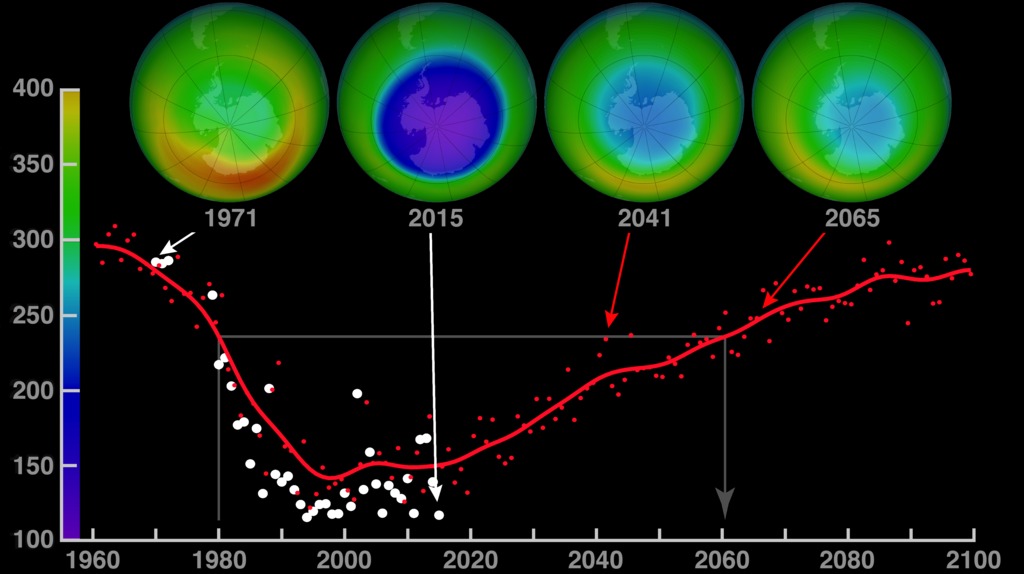
This ozone hole recovery projection extends from 1960 to 2100. Although they dropped dramatically in the 1980s, global ozone levels have stabilized since the mid-1990s. The four globes show monthly-averaged total ozone over Antarctica in October. The 1971 and 2015 globes were created with data from NASA’s Nimbus-4 Backscatter Ultraviolet experiment and the Aura Ozone Monitoring Instrument, respectively. The projections for the 2041 and 2065 globes were made using output from the NASA Goddard Earth Observing System Chemistry-Climate Model (GEOS-CCM). The graph shows each year’s October average minimum ozone (white dots) over Antarctica. The red dots come from the model, and the red curve represents a smoothed version of the dots.
Credit: NASA, public domain, via Wikimedia Commons - Arctic ozone depletion occurs in Northern Hemisphere springtime when strong polar vortices isolate stratospheric circulation, creating PSCs.
- Occasional depletion of ozone has also been noted above the Himalayas.

