
Credit: Gregory Phillips, via Wikimedia Commons
For centuries, humans have relied on pigments to color our products. But a new discovery may change that.
Pigments produce color, ironically, by absorbing light. Chlorophyll, for example, absorbs all colors of the light spectrum except green, which reflects off leaves, making them appear green to our eyes.
Pigments and paints that we manufacture work the same way.
But a few years ago, scientists working to create a perfect mirror out of aluminum noticed that microscopic particles of aluminum oxide clumped on its surface to form dark clouds.
Upon closer inspection, they realized they had created structural color in the oxides.
Structural color can be found in butterfly wings and some feathers, where extremely tiny, even nanoscopic particles determine which wavelengths of light, which colors, reflect back to our eye.
The scientists began experimenting, and by adjusting their process, could produce a multitude of colors.
Their structural-color paint is incredibly lightweight, and the coating incredibly thin.
A few pounds could cover an entire passenger jet—compared to more than a thousand pounds of conventional pigmented paint—saving energy every flight.
What’s more, structural-color paint reflects rather than absorbs heat, which could save huge amounts of energy in cooling and heating.
It’s not commercial yet, but if and when that happens, it could change the way we look at color.
Background
Synopsis: For millennia humans have used pigments that absorb certain wavelengths of light to create colors, but nature has another method of making color. Structural color relies on the physical structure of a material, like butterfly wings, to reflect and scatter light. Recent materials science research accidentally produced this phenomenon using just aluminum and aluminum oxides to produce a range of paint colors that are cooling, nontoxic and remarkably lightweight. While these are not yet available on a commercial scale, once again, nature shows the way!
- The most common source of color is the absorption of certain wavelengths of light by the molecules in pigments, resulting in the transmission of colors that are not absorbed.
- An example is the chlorophyll molecule in plants that absorbs all the wavelengths of white light except green, which is transmitted to our eyes.
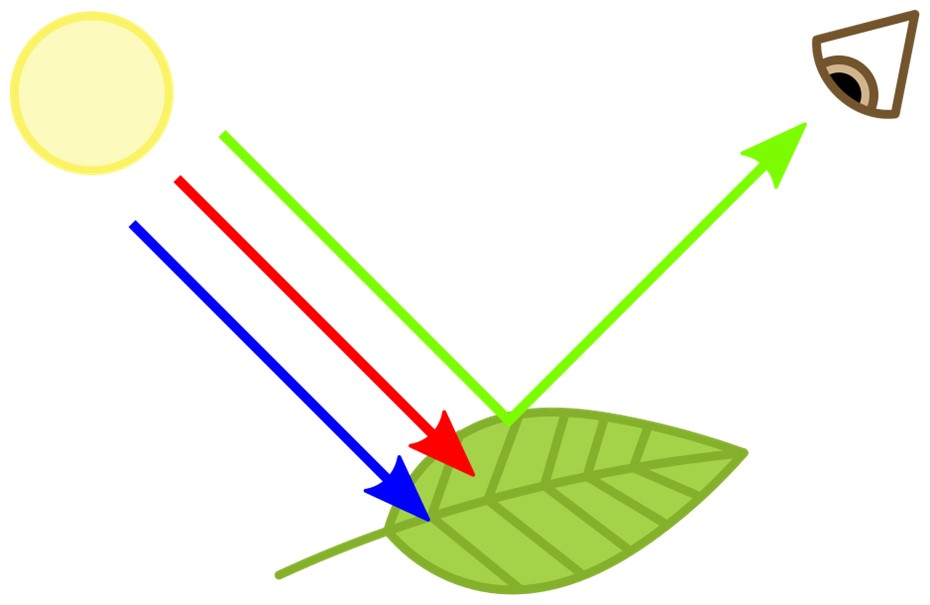
Chlorophyll absorbs red and blue while transmitting green light.
Credit: Nefronus, CC BY-SA 4.0, via Wikimedia Commons - Mineral pigments may come from cobalt (blue), ocher (red) or cadmium (yellow).
- Pigments absorb all the wavelengths of sunlight they do not transmit, so pigments also absorb heat from infrared wavelengths. This is why black cars can heat up as much as 17°F hotter than white cars (ED-281 Super Cool Cities) on a hot summer day.
- Different pigments contain different molecules, each of which produces a unique perceived color by absorbing different amounts of the various colors of the spectrum. Therefore, each paint pigment requires a different formulation, many of which are synthetic and some of which are toxic (especially green pigments).
- An example is the chlorophyll molecule in plants that absorbs all the wavelengths of white light except green, which is transmitted to our eyes.
- In 1665, Robert Hooke described the “fantastical” colors of the peacock’s feathers in his book Micrographia, noting they were composed of a multitude of bright, reflective parts.
- He noted that the colors changed depending on the direction of viewing, tied to alternating reflection or refraction of the light by the microscopic structures.

Robert Hooke's 1665 Micrographia contains the first observations of structural colors.
Credit: Llyfrgell Genedlaethol Cymru–The National Library Of Wales, public domain, via Wikimedia Commons - Hooke found that wetting the iridescent feathers stopped the effect and their color, rendering them brown. Peacock feathers are colored both brown by the pigment melanin and iridescent blue and green by structural color.
- Isaac Newton and Thomas Young wrote about the phenomenon in the 1700s.
- He noted that the colors changed depending on the direction of viewing, tied to alternating reflection or refraction of the light by the microscopic structures.
- The periodic nature of light, typically defined by its wavelength and period, means that optical properties of a material are tied to its microscopic or nanoscopic texture. The secret to structural color is the periodicity of this texture; colors will shift if the spacing changes.
- Structural color, found in butterfly wings, peacock feathers and opals, is created when light interacts with the nanoscale texture of a material to reflect specific wavelengths of light.
- Constructive interference caused by very fine parallel structures on the scale of a color’s wavelength creates structural colors.
- Colors may change when viewed at an angle, which alters the apparent wavelength being transmitted to the viewer’s eye.

This series of scanning electron microscopy (SEM) photographs with increasing magnification (left to right and downward) shows the scale structure of butterfly wings that contribute to structural coloration.
Credit: howcheng, SecretDisc, Michael Apel, and Shaddack, via Wikimedia Commons
This zoned precious opal from the lower Pliocene (4.45 Ma) Opal Mountain rhyolite of the eastern Snake River Plain, Idaho, gets its fantastic color from structural coloration caused by densely stacked layers of quartz nanospheres (ED-301 Outback Opals). Located at the Montana Bureau of Mines and Geology Mineral Museum, Butte, Montana.
Credit: James St. John, via Wikimedia Commons - While some structural coloration produces specific colors, other structural enhancements simply serve to increase brilliance by scattering more light.
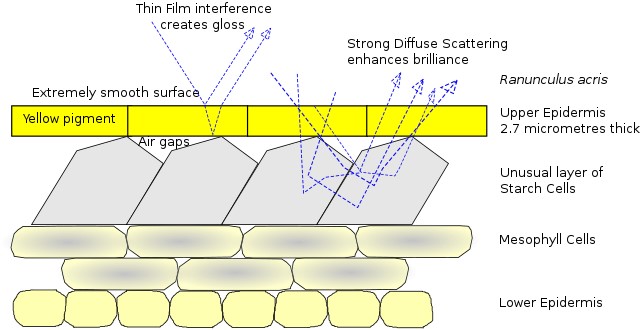
To better attract pollinators, buttercup flowers (Ranunculus acris) take advantage of both pigment and structural coloration to enhance their brilliant yellow color by scattering light. In a similar way, some automobile paint employs structural color to produce a pearlescent effect by the addition of microscopic mica or metallic particles to pigmented paint to scatter light.
Credit: Chiswick Chap, based on van der Kooi, Elzenga, Dijksterhuis, and Stavenga, 2017, CC BY-SA 4.0, via Wikimedia Commons
- While working to produce a flawless mirror, materials scientists at the University of Central Florida accidentally developed a way to produce structural color that may revolutionize the way humans color their world. They call their discovery ultralight plasmonic structural color.
- While using an electron beam evaporator, which heats substances at a precisely controlled rate, to attempt to make a long continuous aluminum mirror, the scientists noticed nanoparticles of aluminum atoms that accumulated on the surface, disrupting the shine of the mirror and putting a dark cloud over their process.
- Then they noticed that different wavelengths of light would cause electrons in the nanoparticles to oscillate depending on the size of the nanoparticles, controlling the color reflected. Out of the cloud came a remarkable silver lining!
- They rallied and developed a paint-like coating made of aluminum and aluminum oxide.
- It is composed of tiny flakes covered with tinier dots of nanoparticles on an oxide substrate. The aluminum atoms prefer each other to the oxide substrate, so they clump into dots.
- The size of the nanoparticle dots on the aluminum flakes determines the color of the coating. Tweaking the temperature and pressure of the electron beam evaporator enables the researchers to control the size of the dots and their resulting color.
- Structural color made with aluminum and aluminum oxide is nontoxic, while heavy metal cobalt- and cadmium-based pigments are toxic.
- All colors can be made from the same material with slightly changed nanoscale textural dimensions, unlike pigments where different base materials are required.
- The nanoparticle coating is extremely lightweight, and only a tiny thickness (150 nanometers) is required to produce the full color effect. A half-teaspoon would cover the front and back of a door.
- Just 3.3 lb (1.3 kg) would cover a Boeing 747 jet, compared to 1,100 lb (500 kg) of pigmented paint. A small tweak in weight can save hundreds of thousands of gallons of jet fuel—and related emissions—per year.

The Boeing 747 Rollout in September 1968.
Credit: SAS Scandinavian Airlines, public domain, via Wikimedia Commons - Structural color doesn’t fade, it is a physical characteristic of the material, so repainting structures and airplanes would not need to occur as frequently.
- It doesn’t absorb sunlight, so it doesn’t trap infrared heat energy like pigments do. It may actually keep surfaces 25°F to 30°F (13–16°C) cooler than traditional paint regardless of the color chosen, creating significant energy savings.
- The researchers now need to scale up the concept to commercial production.
- Ultralight plasmonic structural color provides a vibrant spectrum of cool, reflective, colorful choices.
- On the opposite end of the spectrum, Vantablack (ED-163 Ultra-Black) is a structural color that absorbs 99.96% of incoming light.
- Getting to commercial production will take time, but biomimicry—and a little good luck—has once again shown humans the way.

